A Website Tool for Predicting Triploidy in Larval Fish Spawns Active
Differentiating diploids from triploids at the earliest life stage possible allows for a more efficient use of resources including production time and rearing space. Thus, a reliable flow cytometric (FCM) method has been developed to discriminate triploids from diploids at the larval stage. In order to help simplify the process of differentiating triploids from diploids, we propose a simple website tool called Ploidy Predictor (available at https://warcapps.usgs.gov/gs-eco/warc/ploidy/) to help predict the number of triploid larvae from a spawn after flow cytometric processing.
The Science Issue and Relevance: Triploidy is the condition in which three chromosome sets occur in somatic cells. Triploidization is the most practical, economical, and effective method for mass production of sterile fishes. Some examples include triploid oysters Crassostrea spp., Grass Carp Ctenopharyngodon idella, and Black Carp Mylopharyngodon piceus that are commercially cultured for consumption, weed and snail control, respectively. Additionally, triploid walleye Sander vitreus, crappie Pomoxis spp., striped bass Morone saxatilis, and salmonids are stocked for recreational fishing. Triploidization limits the potential for establishment of wild populations. However, treatments used to induce triploidy often do not achieve 100% triploids in a spawn.
Differentiating diploids from triploids at the earliest life stage possible allows for a more efficient use of resources including production time and rearing space. Thus, a reliable flow cytometric (FCM) method has been developed to discriminate triploids from diploids at the larval stage. In order to help simplify the process of differentiating triploids from diploids, we propose the Ploidy Predictor tool to help predict the number of triploid larvae from a spawn after FCM processing. This tool solves the specific quadratic equations used to predict the percentage of triploid larvae in a pool of either 20 or 50 larvae of unknown ploidy. The tool will increase precision in reading the prediction graphs thereby minimizing human error in solving equations and/or interpreting the graphic displays. This tool will not only allow for exact predictions of the percentage of triploidy in larval spawns, it will simplify the process.

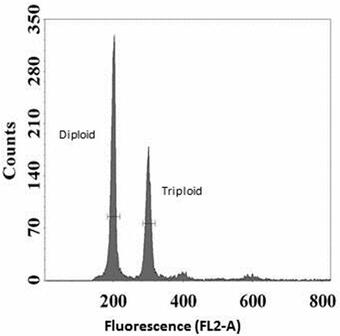
Methodology for Addressing the Issue: At 3 days post-hatch, individual Grass Carp were mechanically disassociated into single-cell suspensions. Nuclear DNA was stained with propidium iodide fluorescent dye and then analyzed by FCM to yield histograms reflecting DNA content, where triploids show nuclei with fluorescence at 1.5 times the diploid level (Fig. 1). Larvae were pooled (n = 20 or 50) per each known triploid/diploid mixture, constituting a set of 15 mixtures from 0 to 100% triploid (Fig. 1). At least 10 replications per known ploidy level were generated to determine the means and variability for the observed FCM triploidy data. Regression analyses generated the best-fitting curves, resulting in a quadratic equation specific for either the pool of 20 or 50 larvae. Thus, an accurate prediction of the proportion of triploids can be generated by following a standard larval processing and FCM analyses, coupled with using the quadratic equation (Table 1) or reading the prediction plot (Fig. 2).
Future Steps: We intend to facilitate the availability of this web-based tool (Fig. 3) to triploid producers, researchers in polyploidy, and state resource agencies. This prediction methodology can be applicable across taxa, using pools of either 20 or 50 larvae from a spawn.
Keywords: Flow cytometry, Asian carp, polyploidy, prediction science, non-linear regression
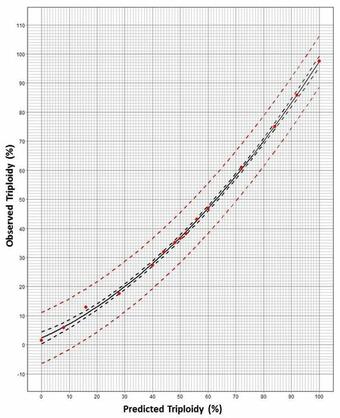
Use of Prediction Plots (Fig. 2): If an observed FCM reading of the area under the triploid curve (Fig. 1) is estimated at 38% triploidy using a subsample of 50 larvae from the spawn, then the triploidy prediction from the graph is 50% in the spawn. The 95% prediction curve range is ~40 - 60%. Alternatively, quadratic equations (Table 1) can be solved for X Prediction (known) using this Y Observed reading (Fig. 3).
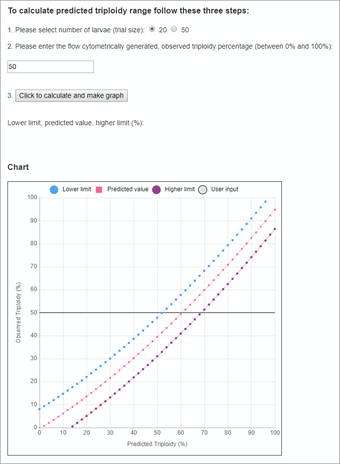
An Interactive Dialogue Box for Estimating Triploidy in a Spawn by using the online Ploidy Predictor (Fig. 3)
- Select how many fish are pooled (20 or 50) from the spawn. Enter the value in the interactive dialogue box.
- Obtain the observed triploidy value from the flow cytometrist and enter this value.
- Click the button to calculate the predicted triploidy percentage range and update the chart. The percentages for the lower calculated limit, actual predicted value, and higher calculated limit values will be displayed.
- The chart offers an alternative presentation of the numerical result. A horizontal line will highlight the observed Y value (flow cytometry data entered at step 2) across the range of predicted curve values: the 95% lower and higher prediction limits and the predicted value.

Below are other science projects associated with this project.
Determining the Ploidy and Resultant Reproductive Capability of Artificially Spawned and Wild Caught Asian Carp
Below are publications associated with this project.
An accurate method for measuring triploidy of larval fish spawns
- Overview
Differentiating diploids from triploids at the earliest life stage possible allows for a more efficient use of resources including production time and rearing space. Thus, a reliable flow cytometric (FCM) method has been developed to discriminate triploids from diploids at the larval stage. In order to help simplify the process of differentiating triploids from diploids, we propose a simple website tool called Ploidy Predictor (available at https://warcapps.usgs.gov/gs-eco/warc/ploidy/) to help predict the number of triploid larvae from a spawn after flow cytometric processing.
 Sources/Usage: Some content may have restrictions. View Media Details
Sources/Usage: Some content may have restrictions. View Media DetailsFig. 1. A flow cytometric histogram of larval Grass Carp cells; 25 larvae were diploid and 25 triploid, for a known triploidy at 50%. The observed triploidy was 38.4%, because tiploid individuals are comprised of fewer cells, with DNA content of triploids being 1.5 times the diploid level. The Science Issue and Relevance: Triploidy is the condition in which three chromosome sets occur in somatic cells. Triploidization is the most practical, economical, and effective method for mass production of sterile fishes. Some examples include triploid oysters Crassostrea spp., Grass Carp Ctenopharyngodon idella, and Black Carp Mylopharyngodon piceus that are commercially cultured for consumption, weed and snail control, respectively. Additionally, triploid walleye Sander vitreus, crappie Pomoxis spp., striped bass Morone saxatilis, and salmonids are stocked for recreational fishing. Triploidization limits the potential for establishment of wild populations. However, treatments used to induce triploidy often do not achieve 100% triploids in a spawn.
Differentiating diploids from triploids at the earliest life stage possible allows for a more efficient use of resources including production time and rearing space. Thus, a reliable flow cytometric (FCM) method has been developed to discriminate triploids from diploids at the larval stage. In order to help simplify the process of differentiating triploids from diploids, we propose the Ploidy Predictor tool to help predict the number of triploid larvae from a spawn after FCM processing. This tool solves the specific quadratic equations used to predict the percentage of triploid larvae in a pool of either 20 or 50 larvae of unknown ploidy. The tool will increase precision in reading the prediction graphs thereby minimizing human error in solving equations and/or interpreting the graphic displays. This tool will not only allow for exact predictions of the percentage of triploidy in larval spawns, it will simplify the process.
 Sources/Usage: Some content may have restrictions. View Media Details
Sources/Usage: Some content may have restrictions. View Media DetailsTable 1. Quadratic equations for predicting the actual triploidy percentage of a spawn using a pool of either 20 or 50 larvae. Methodology for Addressing the Issue: At 3 days post-hatch, individual Grass Carp were mechanically disassociated into single-cell suspensions. Nuclear DNA was stained with propidium iodide fluorescent dye and then analyzed by FCM to yield histograms reflecting DNA content, where triploids show nuclei with fluorescence at 1.5 times the diploid level (Fig. 1). Larvae were pooled (n = 20 or 50) per each known triploid/diploid mixture, constituting a set of 15 mixtures from 0 to 100% triploid (Fig. 1). At least 10 replications per known ploidy level were generated to determine the means and variability for the observed FCM triploidy data. Regression analyses generated the best-fitting curves, resulting in a quadratic equation specific for either the pool of 20 or 50 larvae. Thus, an accurate prediction of the proportion of triploids can be generated by following a standard larval processing and FCM analyses, coupled with using the quadratic equation (Table 1) or reading the prediction plot (Fig. 2).
 Sources/Usage: Some content may have restrictions. View Media Details
Sources/Usage: Some content may have restrictions. View Media DetailsFig. 2. Nonlinear regression prediction plot for use with 50 pooled larvae that accurately estimates the percentage of triploids in a mixed population of triploids and diploids of that spawn. The plot was generated from 10 replicate experiments, each using a FCM protocol for cell processing and analysis at 15 known levels of ploidy. The 95% confidence limits (black dashed lines) and mean values (dots), along with the 95% prediction curves (red dashed lines) are shown. The corresponding quadratic equation is above. Thus, a flow cytometric-derived observed value (y–axis) (within the range) will correspond to a predicted triploidy value (x-axis) for a spawn. Future Steps: We intend to facilitate the availability of this web-based tool (Fig. 3) to triploid producers, researchers in polyploidy, and state resource agencies. This prediction methodology can be applicable across taxa, using pools of either 20 or 50 larvae from a spawn.
Keywords: Flow cytometry, Asian carp, polyploidy, prediction science, non-linear regression
Use of Prediction Plots (Fig. 2): If an observed FCM reading of the area under the triploid curve (Fig. 1) is estimated at 38% triploidy using a subsample of 50 larvae from the spawn, then the triploidy prediction from the graph is 50% in the spawn. The 95% prediction curve range is ~40 - 60%. Alternatively, quadratic equations (Table 1) can be solved for X Prediction (known) using this Y Observed reading (Fig. 3).
 Sources/Usage: Public Domain. View Media Details
Sources/Usage: Public Domain. View Media DetailsFig. 3. A screen shot of the Ploidy Predictor tool, which helps predict the number of triploid larvae from a spawn after flow cytometric processing. An Interactive Dialogue Box for Estimating Triploidy in a Spawn by using the online Ploidy Predictor (Fig. 3)
- Select how many fish are pooled (20 or 50) from the spawn. Enter the value in the interactive dialogue box.
- Obtain the observed triploidy value from the flow cytometrist and enter this value.
- Click the button to calculate the predicted triploidy percentage range and update the chart. The percentages for the lower calculated limit, actual predicted value, and higher calculated limit values will be displayed.
- The chart offers an alternative presentation of the numerical result. A horizontal line will highlight the observed Y value (flow cytometry data entered at step 2) across the range of predicted curve values: the 95% lower and higher prediction limits and the predicted value.
 Sources/Usage: Public Domain. View Media Details
Sources/Usage: Public Domain. View Media DetailsGrass carp larvae of unknown ploidy status. These will be pulled for flow cytometric analysis and estimation of percentage of triploids in the spawn. - Science
Below are other science projects associated with this project.
Determining the Ploidy and Resultant Reproductive Capability of Artificially Spawned and Wild Caught Asian Carp
The invasive grass carp and black carp are artificially spawned to produce triploids, which means they have three sets of chromosomes and are sterile. WARC scientists invented an early ploidy prediction process for produced fry, and a post-mortem assessment method for carp caught either in the wild or sold and hauled live to other states. - Publications
Below are publications associated with this project.
An accurate method for measuring triploidy of larval fish spawns
A standard flow cytometric protocol was developed for estimating triploid induction in batches of larval fish. Polyploid induction treatments are not guaranteed to be 100% efficient, thus the ability to quantify the proportion of triploid larvae generated by a particular treatment helps managers to stock high-percentage spawns and researchers to select treatments for efficient triploid induction.AuthorsJill A. Jenkins, Rassa O. Draugelis-Dale, Robert Glennon, Anita M. Kelly, Bonnie L. Brown, John Morrison


