Picture of Cape Alava at sunset. Cape Alava is a point of land on the outer coast of the Olympic Peninsula. The cape is situated within Olympic National Park, and the Makah Indian Reservation.
Carla M Conway
Carla is a Biological Science Laboratory Technician (Microbiology) at the Western Fisheries Research Center.
Science and Products
Non-lethal Detection of Skin Injuries in Juvenile Chinook Salmon Oncorhynchus tshawytscha by Fast Green FCF Dye
Physiological and molecular endpoints observed in juvenile largemouth bass in response to an estrogen (17α-ethinylestradiol) and subsequently a bacterial challenge (Edwardsiella piscicida) exposure under laboratory conditions. Physiological and molecular endpoints observed in juvenile largemouth bass in response to an estrogen (17α-ethinylestradiol) and subsequently a bacterial challenge (Edwardsiella piscicida) exposure under laboratory conditions.
Histological and molecular testing of nuclear inclusion X in Pacific Razor clams from select locations in Washington, USA Histological and molecular testing of nuclear inclusion X in Pacific Razor clams from select locations in Washington, USA
Pufferfish mortality data Pufferfish mortality data
Picture of Cape Alava at sunset. Cape Alava is a point of land on the outer coast of the Olympic Peninsula. The cape is situated within Olympic National Park, and the Makah Indian Reservation.
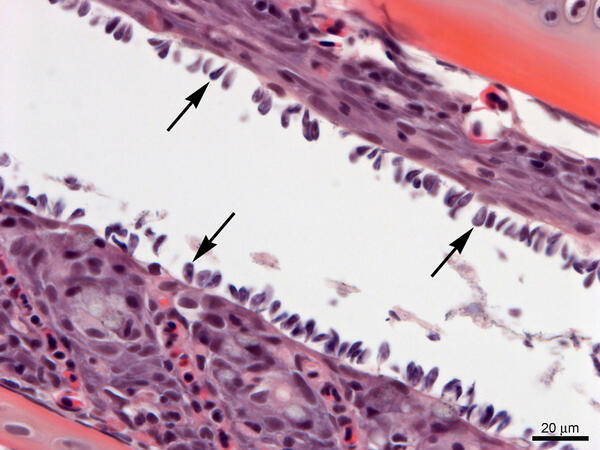
Gills from Lost River Suckers - heavy infestation of Ichthyobodo sp.
Gills from Lost River Suckers - heavy infestation of Ichthyobodo sp.Gills from Lost River suckers with a heavy infestation of Ichthyobodo sp. (arrows). Slide is stained with hematoxylin and eosin.
Gills from Lost River Suckers - heavy infestation of Ichthyobodo sp.
Gills from Lost River Suckers - heavy infestation of Ichthyobodo sp.Gills from Lost River suckers with a heavy infestation of Ichthyobodo sp. (arrows). Slide is stained with hematoxylin and eosin.

WFRC DFAT for detection of Renibacterium salmoninarum
WFRC DFAT for detection of Renibacterium salmoninarumDirect fluorescent antibody test (DFAT) for the detection of Renibacterium salmoninarum in tissues. Fluorescing R. salmoninarum cells are visible on a slide.
WFRC DFAT for detection of Renibacterium salmoninarum
WFRC DFAT for detection of Renibacterium salmoninarumDirect fluorescent antibody test (DFAT) for the detection of Renibacterium salmoninarum in tissues. Fluorescing R. salmoninarum cells are visible on a slide.

Figure 1. Appearance of Descaling Site Exposed to Fast Green FCF Dye
Figure 1. Appearance of Descaling Site Exposed to Fast Green FCF DyeFigure 1. Appearance of descaling site exposed to fast green FCF dye six hours after intentional descaling injury, showing loss of scales and presence of fast green staining. Areas of unintentional integumental injury are also stained (arrows).
Related image Figure 2.
Figure 1. Appearance of Descaling Site Exposed to Fast Green FCF Dye
Figure 1. Appearance of Descaling Site Exposed to Fast Green FCF DyeFigure 1. Appearance of descaling site exposed to fast green FCF dye six hours after intentional descaling injury, showing loss of scales and presence of fast green staining. Areas of unintentional integumental injury are also stained (arrows).
Related image Figure 2.

Figure 2. Scanning Electron Micrograph of Descaling Area
Figure 2. Scanning Electron Micrograph of Descaling AreaFigure 2. Scanning electron micrograph of descaling area delimited by box in Figure 1 showing epidermal disruption, empty scale pockets and an exposed scale with visible concentric ridges (upper right). Scale bar = 500 µm.
Figure 2. Scanning Electron Micrograph of Descaling Area
Figure 2. Scanning Electron Micrograph of Descaling AreaFigure 2. Scanning electron micrograph of descaling area delimited by box in Figure 1 showing epidermal disruption, empty scale pockets and an exposed scale with visible concentric ridges (upper right). Scale bar = 500 µm.

Figure 3. Appearance of Descaling Site Exposed to Fast Green FCF Dye
Figure 3. Appearance of Descaling Site Exposed to Fast Green FCF DyeFigure 3. Appearance of descaling site exposed to fast green FCF dye 96 hours after intentional descaling injury, showing lack of scales, presence of fast green staining in areas of epidermal disruption and absence of staining in areas where migrating epidermal cells have closed the wound.
Figure 3. Appearance of Descaling Site Exposed to Fast Green FCF Dye
Figure 3. Appearance of Descaling Site Exposed to Fast Green FCF DyeFigure 3. Appearance of descaling site exposed to fast green FCF dye 96 hours after intentional descaling injury, showing lack of scales, presence of fast green staining in areas of epidermal disruption and absence of staining in areas where migrating epidermal cells have closed the wound.

Figure 4. Scanning Electron Micrograph of Descaling Area
Figure 4. Scanning Electron Micrograph of Descaling AreaFigure 4. Scanning electron micrograph of descaling area delimited by box in Figure 3 showing epidermal disruption (arrows), empty scale pockets and restoration of epidermal integrity (asterisk). An exposed scale with visible concentric ridges is visible at the lower center. Scale bar = 500 µm.
Figure 4. Scanning Electron Micrograph of Descaling Area
Figure 4. Scanning Electron Micrograph of Descaling AreaFigure 4. Scanning electron micrograph of descaling area delimited by box in Figure 3 showing epidermal disruption (arrows), empty scale pockets and restoration of epidermal integrity (asterisk). An exposed scale with visible concentric ridges is visible at the lower center. Scale bar = 500 µm.
Host jump of an exotic fish rhabdovirus into a new class of animals poses a disease threat to amphibians Host jump of an exotic fish rhabdovirus into a new class of animals poses a disease threat to amphibians
Temporal, environmental, and demographic correlates of Ichthyophonus sp. infections in mature Pacific herring populations Temporal, environmental, and demographic correlates of Ichthyophonus sp. infections in mature Pacific herring populations
Exposure to 17α-ethinylestradiol results in differential susceptibility of largemouth bass (Micropterus salmoides) to bacterial infection Exposure to 17α-ethinylestradiol results in differential susceptibility of largemouth bass (Micropterus salmoides) to bacterial infection
Evaluating the effect of nuclear inclusion X (NIX) infections on Pacific razor clam populations Evaluating the effect of nuclear inclusion X (NIX) infections on Pacific razor clam populations
Disruption of the Francisella noatunensis orientalis pdpA gene results in virulence attenuation and protection in zebrafish Disruption of the Francisella noatunensis orientalis pdpA gene results in virulence attenuation and protection in zebrafish
Survival and growth of suckers in mesocosms at three locations within Upper Klamath Lake, Oregon, 2018 Survival and growth of suckers in mesocosms at three locations within Upper Klamath Lake, Oregon, 2018
Differential susceptibility of Yukon River and Salish Sea stocks of Chinook salmon Oncorhynchus tshawytscha to ichthyophoniasis Differential susceptibility of Yukon River and Salish Sea stocks of Chinook salmon Oncorhynchus tshawytscha to ichthyophoniasis
Novel diagnostic tests for the putative agent of bacterial gill disease in Pacific razor clams (Siliqua patula) Novel diagnostic tests for the putative agent of bacterial gill disease in Pacific razor clams (Siliqua patula)
Consequences of Piscine orthoreovirus genotype 1 (PRV‐1) infections in Chinook salmon (Oncorhynchus tshawytscha ), coho salmon (O. kisutch ) and rainbow trout (O. mykiss ) Consequences of Piscine orthoreovirus genotype 1 (PRV‐1) infections in Chinook salmon (Oncorhynchus tshawytscha ), coho salmon (O. kisutch ) and rainbow trout (O. mykiss )
Mortality of endangered juvenile Lost River Suckers associated with cyanobacteria blooms in mesocosms in Upper Klamath Lake, Oregon Mortality of endangered juvenile Lost River Suckers associated with cyanobacteria blooms in mesocosms in Upper Klamath Lake, Oregon
Effects of microcystin-LR on juvenile Lost River suckers (Deltistes luxatus) during feeding trials, Upper Klamath Lake, Oregon, 2014−16 Effects of microcystin-LR on juvenile Lost River suckers (Deltistes luxatus) during feeding trials, Upper Klamath Lake, Oregon, 2014−16
Assessing causes of mortality for endangered juvenile Lost River suckers (Deltistes luxatus) in mesocosms in Upper Klamath Lake, south-central Oregon, 2016 Assessing causes of mortality for endangered juvenile Lost River suckers (Deltistes luxatus) in mesocosms in Upper Klamath Lake, south-central Oregon, 2016
Science and Products
Non-lethal Detection of Skin Injuries in Juvenile Chinook Salmon Oncorhynchus tshawytscha by Fast Green FCF Dye
Physiological and molecular endpoints observed in juvenile largemouth bass in response to an estrogen (17α-ethinylestradiol) and subsequently a bacterial challenge (Edwardsiella piscicida) exposure under laboratory conditions. Physiological and molecular endpoints observed in juvenile largemouth bass in response to an estrogen (17α-ethinylestradiol) and subsequently a bacterial challenge (Edwardsiella piscicida) exposure under laboratory conditions.
Histological and molecular testing of nuclear inclusion X in Pacific Razor clams from select locations in Washington, USA Histological and molecular testing of nuclear inclusion X in Pacific Razor clams from select locations in Washington, USA
Pufferfish mortality data Pufferfish mortality data
Picture of Cape Alava at sunset. Cape Alava is a point of land on the outer coast of the Olympic Peninsula. The cape is situated within Olympic National Park, and the Makah Indian Reservation.
Picture of Cape Alava at sunset. Cape Alava is a point of land on the outer coast of the Olympic Peninsula. The cape is situated within Olympic National Park, and the Makah Indian Reservation.

Gills from Lost River Suckers - heavy infestation of Ichthyobodo sp.
Gills from Lost River Suckers - heavy infestation of Ichthyobodo sp.Gills from Lost River suckers with a heavy infestation of Ichthyobodo sp. (arrows). Slide is stained with hematoxylin and eosin.
Gills from Lost River Suckers - heavy infestation of Ichthyobodo sp.
Gills from Lost River Suckers - heavy infestation of Ichthyobodo sp.Gills from Lost River suckers with a heavy infestation of Ichthyobodo sp. (arrows). Slide is stained with hematoxylin and eosin.

WFRC DFAT for detection of Renibacterium salmoninarum
WFRC DFAT for detection of Renibacterium salmoninarumDirect fluorescent antibody test (DFAT) for the detection of Renibacterium salmoninarum in tissues. Fluorescing R. salmoninarum cells are visible on a slide.
WFRC DFAT for detection of Renibacterium salmoninarum
WFRC DFAT for detection of Renibacterium salmoninarumDirect fluorescent antibody test (DFAT) for the detection of Renibacterium salmoninarum in tissues. Fluorescing R. salmoninarum cells are visible on a slide.

Figure 1. Appearance of Descaling Site Exposed to Fast Green FCF Dye
Figure 1. Appearance of Descaling Site Exposed to Fast Green FCF DyeFigure 1. Appearance of descaling site exposed to fast green FCF dye six hours after intentional descaling injury, showing loss of scales and presence of fast green staining. Areas of unintentional integumental injury are also stained (arrows).
Related image Figure 2.
Figure 1. Appearance of Descaling Site Exposed to Fast Green FCF Dye
Figure 1. Appearance of Descaling Site Exposed to Fast Green FCF DyeFigure 1. Appearance of descaling site exposed to fast green FCF dye six hours after intentional descaling injury, showing loss of scales and presence of fast green staining. Areas of unintentional integumental injury are also stained (arrows).
Related image Figure 2.

Figure 2. Scanning Electron Micrograph of Descaling Area
Figure 2. Scanning Electron Micrograph of Descaling AreaFigure 2. Scanning electron micrograph of descaling area delimited by box in Figure 1 showing epidermal disruption, empty scale pockets and an exposed scale with visible concentric ridges (upper right). Scale bar = 500 µm.
Figure 2. Scanning Electron Micrograph of Descaling Area
Figure 2. Scanning Electron Micrograph of Descaling AreaFigure 2. Scanning electron micrograph of descaling area delimited by box in Figure 1 showing epidermal disruption, empty scale pockets and an exposed scale with visible concentric ridges (upper right). Scale bar = 500 µm.

Figure 3. Appearance of Descaling Site Exposed to Fast Green FCF Dye
Figure 3. Appearance of Descaling Site Exposed to Fast Green FCF DyeFigure 3. Appearance of descaling site exposed to fast green FCF dye 96 hours after intentional descaling injury, showing lack of scales, presence of fast green staining in areas of epidermal disruption and absence of staining in areas where migrating epidermal cells have closed the wound.
Figure 3. Appearance of Descaling Site Exposed to Fast Green FCF Dye
Figure 3. Appearance of Descaling Site Exposed to Fast Green FCF DyeFigure 3. Appearance of descaling site exposed to fast green FCF dye 96 hours after intentional descaling injury, showing lack of scales, presence of fast green staining in areas of epidermal disruption and absence of staining in areas where migrating epidermal cells have closed the wound.

Figure 4. Scanning Electron Micrograph of Descaling Area
Figure 4. Scanning Electron Micrograph of Descaling AreaFigure 4. Scanning electron micrograph of descaling area delimited by box in Figure 3 showing epidermal disruption (arrows), empty scale pockets and restoration of epidermal integrity (asterisk). An exposed scale with visible concentric ridges is visible at the lower center. Scale bar = 500 µm.
Figure 4. Scanning Electron Micrograph of Descaling Area
Figure 4. Scanning Electron Micrograph of Descaling AreaFigure 4. Scanning electron micrograph of descaling area delimited by box in Figure 3 showing epidermal disruption (arrows), empty scale pockets and restoration of epidermal integrity (asterisk). An exposed scale with visible concentric ridges is visible at the lower center. Scale bar = 500 µm.



