ANOVA with post hoc multiple pairwise statistical comparison
Detailed Description
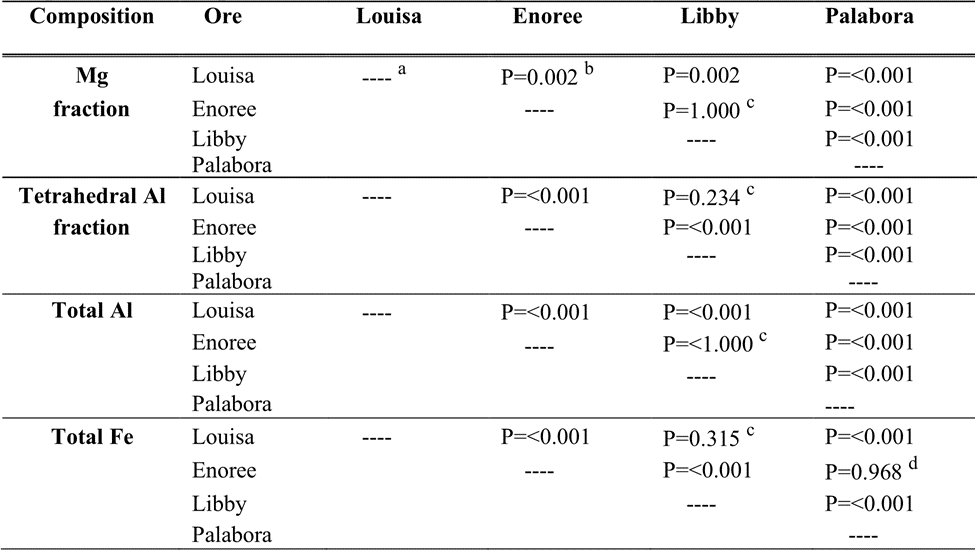
Table A1. ANOVA with post hoc multiple pairwise statistical comparison between compositions of unexpanded vermiculite ore samples examined in this study.
Notes: Mg fraction = Mg/(Mg+Aloct +Feoct), tetrahedral Al fraction = (Altet + Si), total Al, and total Fe are based on Electron Probe MicroAnalysis (EPMA) of the unexpanded ore samples studied from each of the four major historical sources. Mg=total Mg; oct = octahedral; tet = tetrahedral.
a ---- = test not conducted.
b P is used as an ANOVA (Analysis of Variability) statistical test metric of whether differences in median compositional values of the unexpanded ores are statistically distinct from random sampling variability. A value of P < 0.050 is evidence that the median compositions of a group of samples are statistically distinct from the median compositions of the other group of samples. A value of P > 0.050 is weak evidence that the median compositions of a group of samples are statistically distinct from median compositions of the other group of samples. Differences may be due to random variations in composition. A value of P close to 0.05 indicates that the median composition of a group of samples may or may not be statistically distinct from the median compositions of the other group of samples. A value of P near 0.001 provides strong evidence that the median compositions of a group of samples are statistically distinct from the median composition of the other group of samples. ANOVA test on ranks performed and multiple pairwise comparisons done using Dunn’s test.
c Median EPMA compositional values of the unexpanded Louisa, Enoree, and Libby samples are statistically distinct from each other except for the Mg fraction and total Al in the unexpanded Enoree and Libby samples, and the tetrahedral Al fraction and total Fe in the unexpanded Louisa and Libby samples, which are statistically similar.
Sources/Usage
Public Domain.
Characterizing the source of potentially asbestos-bearing commercial vermiculite insulation using in situ IR spectroscopy
Swayze, G.A., Lowers, H.A., Benzel, W.M., Clark, R.N., Driscoll, R.L., Perlman, Z.S., Hoefen, T.M., and Dyar, M.D., 2018, American Mineralogist, v. 103, p. 517-549.
https://doi.org/10.2138/am-2018-6022
Abstract:
Commercially produced vermiculite insulation from Libby, Montana, contains trace
levels of asbestiform amphibole, which is known to cause asbestos-related diseases. When
vermiculite insulation is found in a building, evaluation for its potential asbestos content
traditionally involves collecting a sample from an attic or wall and submitting it for potentially
time-consuming analyses at an off-site laboratory. The goal of this study was to determine if in
situ near-infrared reflectance measurements could be used to reliably identify the source of
vermiculite ore and therefore its potential to contain asbestos. Spectra of 52 expanded ore
samples, including attic insulation, commercial packing materials, and horticultural products
from Libby, Montana; Louisa, Virginia; Enoree, South Carolina; Palabora, South Africa; and
Jiangsu, China, were measured with a portable spectrometer. The mine sources for these
vermiculite ores were identified based on collection location, when known, and on differences in
elemental composition as measured by electron probe microanalysis. Reflectance spectra of the
insulation samples show vibrational overtone and combination absorptions that vary in
wavelength position and relative intensity depending on elemental composition and proportions
of their constituent micas (i.e., vermiculite ore usually consists of a mixture of hydrobiotite and
vermiculite mineral flakes). Band depth ratios of the 1.38/2.32-, 1.40/1.42-, and 2.24/2.38-µm
absorptions allow determination of a vermiculite insulation’s source and detection of its potential
to contain amphibole, talc, and/or serpentine impurities. Spectroscopy cannot distinguish
asbestiform vs non-asbestiform amphiboles. However, if the spectrally determined mica
composition and mineralogy of an insulation sample is consistent with ore from Libby, then it is
likely that some portion of the sodic-calcic amphibole it contains is asbestiform, given that all of
the nearly two dozen Libby vermiculite insulation samples examined with scanning electron
microscopy in this study contain amphiboles. One sample of expanded vermiculite ore from
multiple sources was recognized as a limitation of the spectral method, therefore an additional
test (i.e., 2.24-µm absorption position vs 2.24/2.38-µm band depth) was incorporated into the
spectral method to eliminate misclassification caused by such mixtures. With portable field
spectrometers, the methodology developed can be used to determine vermiculite insulation’s
source and estimate its potential amphibole content, thereby providing low-cost analysis with
onsite reporting to property owners.

