Plants and trees couldn't thrive without capillary action. Capillary action helps bring water up into the roots. With the help of adhesion and cohesion, water can work its way all the way up to the branches and leaves. Read on to learn more about how this movement of water takes place.
• Water Science School HOME • Water Properties topics •
Capillary Action

Credit: Dr. Keith Hayward
Even if you've never heard of capillary action, it is still important in your life. Capillary action is important for moving water (and all of the things that are dissolved in it) around. It is defined as the movement of water within the spaces of a porous material due to the forces of adhesion, cohesion, and surface tension.
Capillary action occurs because water is sticky, thanks to the forces of cohesion (water molecules like to stay close together) and adhesion (water molecules are attracted and stick to other substances). Adhesion of water to the walls of a vessel will cause an upward force on the liquid at the edges and result in a meniscus which turns upward. The surface tension acts to hold the surface intact. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. The height to which capillary action will take water in a uniform circular tube (picture to right) is limited by surface tension and, of course, gravity.
Not only does water tend to stick together in a drop, it sticks to glass, cloth, organic tissues, soil, and, luckily, to the fibers in a paper towel. Dip a paper towel into a glass of water and the water will "climb" onto the paper towel. In fact, it will keep going up the towel until the pull of gravity is too much for it to overcome.
Capillary action is all around us every day

- If you dip a paper towel in water, you will see it "magically" climb up the towel, appearing to ignore gravity. You are seeing capillary action in action, and "climbing up" is about right - the water molecules climb up the towel and drag other water molecules along. (Obviously, Mona Lisa is a big fan of capillary action!)
- Plants and trees couldn't thrive without capillary action. Plants put down roots into the soil which are capable of carrying water from the soil up into the plant. Water, which contains dissolved nutrients, gets inside the roots and starts climbing up the plant tissue. Capillary action helps bring water up into the roots. But capillary action can only "pull" water up a small distance, after which it cannot overcome gravity. To get water up to all the branches and leaves, the forces of adhesion and cohesion go to work in the plant's xylem to move water to the furthest leaf.
- Capillary action is also essential for the drainage of constantly produced tear fluid from the eye. Two tiny-diameter tubes, the lacrimal ducts, are present in the inner corner of the eyelid; these ducts secrete tears into the eye. (Source: Wikipedia)
- Maybe you've used a fountain pen .... or maybe your parents or grandparents did. The ink moves from a reservoir in the body of the pen down to the tip and into the paper (which is composed of tiny paper fibers and air spaces between them), and not just turning into a blob. Of course gravity is responsible for the ink moving "downhill" to the pen tip, but capillary action is needed to keep the ink flowing onto the paper.
The proof is in the pudding ... I mean, in the celery

You can see capillary action in action (although slowly) by doing an experiment where you place the bottom of a celery stalk in a glass of water with food coloring and watch for the movement of the color to the top leaves of the celery. You might want to use a piece of celery that has begun to whither, as it is in need of a quick drink. It can take a few days, but, as these pictures show, the colored water is "drawn" upward, against the pull of gravity. This effect happens because, in plants, water molecules move through narrow tubes that are called capillaries (or xylem).

Do you think you know a lot about water properties?
Take our interactive water-properties true/false quiz and test your water knowledge.
Sources and more information
Learn more about capillary action and related water properties.
Water Properties Information by Topic
Water Meniscus
Surface Tension and Water
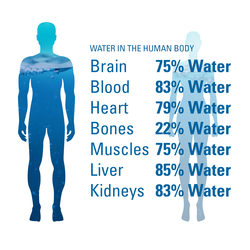
The Water in You: Water and the Human Body
Water Properties True/False Quiz
Adhesion and Cohesion of Water
Below are multimedia items associated with capillary action and related water properties.
Below are publications associated with capillary action and related water properties.
Soil water retention and maximum capillary drive from saturation to oven dryness Soil water retention and maximum capillary drive from saturation to oven dryness
Plants and trees couldn't thrive without capillary action. Capillary action helps bring water up into the roots. With the help of adhesion and cohesion, water can work its way all the way up to the branches and leaves. Read on to learn more about how this movement of water takes place.
• Water Science School HOME • Water Properties topics •
Capillary Action

Credit: Dr. Keith Hayward
Even if you've never heard of capillary action, it is still important in your life. Capillary action is important for moving water (and all of the things that are dissolved in it) around. It is defined as the movement of water within the spaces of a porous material due to the forces of adhesion, cohesion, and surface tension.
Capillary action occurs because water is sticky, thanks to the forces of cohesion (water molecules like to stay close together) and adhesion (water molecules are attracted and stick to other substances). Adhesion of water to the walls of a vessel will cause an upward force on the liquid at the edges and result in a meniscus which turns upward. The surface tension acts to hold the surface intact. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. The height to which capillary action will take water in a uniform circular tube (picture to right) is limited by surface tension and, of course, gravity.
Not only does water tend to stick together in a drop, it sticks to glass, cloth, organic tissues, soil, and, luckily, to the fibers in a paper towel. Dip a paper towel into a glass of water and the water will "climb" onto the paper towel. In fact, it will keep going up the towel until the pull of gravity is too much for it to overcome.
Capillary action is all around us every day

- If you dip a paper towel in water, you will see it "magically" climb up the towel, appearing to ignore gravity. You are seeing capillary action in action, and "climbing up" is about right - the water molecules climb up the towel and drag other water molecules along. (Obviously, Mona Lisa is a big fan of capillary action!)
- Plants and trees couldn't thrive without capillary action. Plants put down roots into the soil which are capable of carrying water from the soil up into the plant. Water, which contains dissolved nutrients, gets inside the roots and starts climbing up the plant tissue. Capillary action helps bring water up into the roots. But capillary action can only "pull" water up a small distance, after which it cannot overcome gravity. To get water up to all the branches and leaves, the forces of adhesion and cohesion go to work in the plant's xylem to move water to the furthest leaf.
- Capillary action is also essential for the drainage of constantly produced tear fluid from the eye. Two tiny-diameter tubes, the lacrimal ducts, are present in the inner corner of the eyelid; these ducts secrete tears into the eye. (Source: Wikipedia)
- Maybe you've used a fountain pen .... or maybe your parents or grandparents did. The ink moves from a reservoir in the body of the pen down to the tip and into the paper (which is composed of tiny paper fibers and air spaces between them), and not just turning into a blob. Of course gravity is responsible for the ink moving "downhill" to the pen tip, but capillary action is needed to keep the ink flowing onto the paper.
The proof is in the pudding ... I mean, in the celery

You can see capillary action in action (although slowly) by doing an experiment where you place the bottom of a celery stalk in a glass of water with food coloring and watch for the movement of the color to the top leaves of the celery. You might want to use a piece of celery that has begun to whither, as it is in need of a quick drink. It can take a few days, but, as these pictures show, the colored water is "drawn" upward, against the pull of gravity. This effect happens because, in plants, water molecules move through narrow tubes that are called capillaries (or xylem).

Do you think you know a lot about water properties?
Take our interactive water-properties true/false quiz and test your water knowledge.
Sources and more information
Learn more about capillary action and related water properties.
Water Properties Information by Topic
Water Meniscus
Surface Tension and Water
The Water in You: Water and the Human Body
Water Properties True/False Quiz
Adhesion and Cohesion of Water
Below are multimedia items associated with capillary action and related water properties.
Below are publications associated with capillary action and related water properties.