Making Minerals-How Growing Rocks Can Help Reduce Carbon Emissions
How Growing Rocks Can Help Reduce Carbon Emissions
Following an assessment of geologic carbon storage potential in sedimentary rocks, the USGS has published a comprehensive review of potential carbon storage in igneous and metamorphic rocks through a process known as carbon mineralization.
As concentrations of carbon dioxide increase in the atmosphere, attention is now being paid to the benefits of removing and storing it in the first place - a process known as carbon dioxide storage.
In 2013, USGS released the first-ever comprehensive national assessment of geologic carbon dioxide storage potential in sedimentary basins. According to this assessment, the United States could store up to 3,000 metric gigatons of carbon dioxide. Now, the USGS has published a comprehensive review of another type of geologic carbon storage: carbon mineralization.
Making Minerals
Carbon mineralization is the process by which carbon dioxide becomes a solid mineral, such as a carbonate. It is a chemical reaction that happens when certain rocks are exposed to carbon dioxide. The biggest advantage of carbon mineralization is that the carbon cannot escape back to the atmosphere.
It happens naturally, but the process can be sped up artificially. Most of the rocks that have the potential for carbon mineralization are igneous or metamorphic, as opposed to porous sedimentary reservoirs.
The primary difference between carbon storage in sedimentary reservoirs and carbon mineralization is that in the sedimentary reservoirs, the injected carbon dioxide dissolves into deep saline groundwaters. However, in carbon mineralization, chemical reactions form a new carbonate mineral within the rocks it is meant to be stored in, preventing possible escape later.
There are two primary types of geologic carbon mineralization: injection of carbon dioxide into rock formations deep underground, or exposure to broken pieces of rock at the surface, such as leftovers from mining, called mine tailings.
Injecting Carbon Deep Underground
This method of carbon mineralization is most similar to geologic carbon storage in sedimentary basins. The carbon dioxide is injected into wells that go deep underground to igneous or metamorphic rock formations that have the potential for carbon mineralization.
The two primary rock types that have the potential for carbon mineralization through injection are basalt and a broad category of rocks called ultramafic, meaning they have extremely high amounts of magnesium and iron. Laboratory studies have shown that ultramafic rocks have the fastest reaction times, and pilot studies have shown that injection of carbon dioxide into basalt can lead to mineralization in under two years.
Mineralizing Carbon with Crushed Rocks
Meanwhile, back at the surface, the other method of carbon mineralization involves exposing carbon dioxide to ultramafic rocks or basalt at the surface. Often these rocks are in the form of crushed mining waste, such as asbestos mine tailings. Carbon mineralization of asbestos mine tailings would have the added benefit of reducing the risks associated with exposed asbestos.
Carbon mineralization of mine waste can be a much faster process than injecting the carbon underground for mineralization, since there is more surface area on the crushed rocks for the carbon to form minerals. However, there is not nearly as much rock that can be mineralized on the surface as there is underground, so the overall amount of carbon storage is higher for underground injection than exposing carbon dioxide to crushed rock on the surface. Likely the best use for this method would be close to industrial sites with carbon dioxide emissions, where the carbon could be captured before it goes into the atmosphere and immediately mineralized onsite.
Carbon Cost Comparisons
Carbon mineralization is but one method of geologic carbon storage, and which method gets chosen for each situation will depend on a variety of factors. One of the most important factors, though, will be the cost per ton to store that carbon.
Currently, storing carbon in sedimentary basins is the most cost-effective method, assuming the amount of pressure in the basin does not reduce the storage space that would otherwise be available. Storage in brine-filled sedimentary reservoirs could cost about \$7-13 per metric ton of carbon dioxide. However, conditions tend to vary significantly throughout sedimentary basins, and some management of pressure and water is likely to be required, which could increase the cost to around \$20-80 per metric ton of carbon dioxide.
Meanwhile, carbon mineralization of crushed rocks at the surface, such as mine tailings or industrial waste, has been estimated to cost around $8 per metric ton of carbon dioxide. However, this is only cost-effective at the local scale and for already mined materials. If mining is required, the cost increases significantly.
Based on limited results from a few pilot projects, carbon mineralization in deep underground basaltic formations could be around \$30 per metric ton of carbon dioxide. No estimates have been made yet for storage in ultramafic rock formations.
The cost benefit analysis suggests that perhaps the most effective use for carbon mineralization is as an option to complement sedimentary brine carbon storage.
All Across America
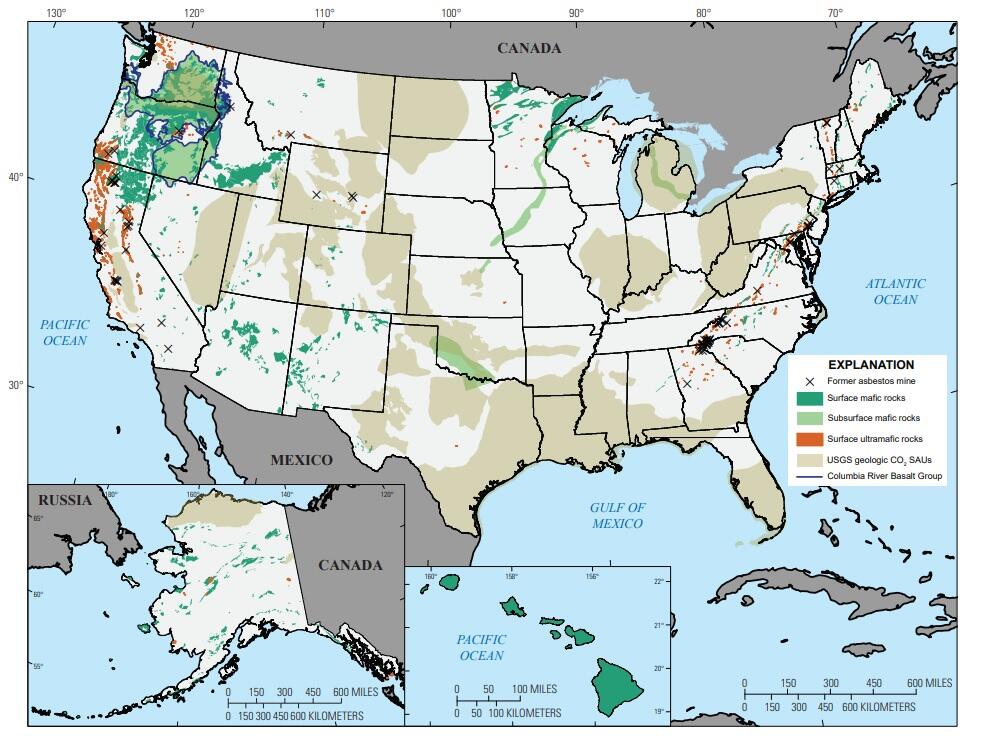
Just as with the geologic carbon storage in sedimentary basins studied previously, the potential for carbon storage through mineralization is spread throughout the United States, though in markedly different locations. There are a few hot spots that deserve special mention.
The biggest hot spot is in the Pacific Northwest. The Columbia River Basalts within Idaho, Oregon and Washington have a large amount of potential, both at the surface and underground.
Another region with abundant basalts, though most are underground and are of uncertain quality, is the midcontinent from Minnesota, Wisconsin, and Michigan, all the way down to Oklahoma and Texas.
Almost the entire state of Hawai’i, being made up of volcanic basalt, has potential both for surface mineralization and injection deep underground.
For surface storage, ultramafic rocks and mine tailings exist over widespread regions within both the east and west coast states. There are also a number of areas with potential in the upper Midwest.
Risks
No industrial process is without potential hazards, and the methods of carbon mineralization described above do have some. The most significant is probably the potential for triggering earthquakes with the injection of carbon underground for mineralization. This potential hazard is mostly dependent on the likelihood of the interaction between existing faults and altering the current pressure in the rock formation by injecting the carbon dioxide.
Other environmental hazards include negatively impacting ecosystems both underground and at the surface, as well as large water requirements for these processes. Future research will continue to fill in gaps about potential environmental concerns as well as resource potential for mineralization.
Start with Science
Just as there are many sources of carbon emissions, there are many potential methods for storing those carbon emissions. In addition, these methods range in scale from individual sites at the local level to large-scale regional storage methods.
Carbon mineralization is one such method that has great potential at the local, site specific level. In addition, the United States has resources throughout the country that can enable mineralization at a variety of sites.
Addressing greenhouse gas emissions and their effects will continue to be a great challenge for this country, as well as the rest of the world. Research like this into carbon mineralization, though, represents one of the best methods for acquiring tools to address that challenge.