Synthetic Biology Tools for Controlling Invasive Carps
We aim to develop control tools for eliminating invasive carps (silver carp – Hypophthalmichthys molitrix, bighead carp – H. nobilis, grass carp – Ctenopharyngodon idella, and black carp – Mylopharyngodon piceus) that use natural gene regulation mechanisms (RNA-induced gene silencing; RNA interference; RNAi) to be species-specific without non-target effects. Invasive carps cause substantial economic and ecological damage, harming valuable native fisheries while having negligible value themselves.

There are a few options available for controlling invasive carps, but their usage can vary in effectiveness or cause their own ecological damage. With chemical piscicides, there is a delicate balancing act to apply a dose that is lethal for the target organism but tolerable for others. Genetic control tools are another option that has the potential to have greater species-specificity meaning they are harmless to anything else cohabitating with the target species.
There are a couple of genetic control tools managers can use to prevent the spread of invasive carp. One strategy is to use a gene drive to spread a negative trait through a population, such as female sterility. Using one of a few molecular biology techniques (e.g. CRISPR), we can guarantee that defective alleles (version of a gene that does not work properly) will be transferred to all offspring. This means a few genetically modified individuals could be released to mate with wild-type animals (e.g. invasive carp) and change the genetic makeup of a population so that it is no longer viable resulting in population crash. Another strategy is to use RNA interference (RNAi) to selectively turn off critical genes of the target organism. This strategy takes advantage of the natural gene regulation mechanism called RNA-induced gene silencing in which small interfering RNAs (siRNAs), short pieces of RNA, guide destruction of other mRNA molecules. Targeting unique sequences that do not occur in other species allows us to turn off critical genes causing mortality while being harmless to any other species that live in the same water.
Both above strategies have advantages and disadvantages. Gene drives will require less application effort because it involves releasing a few animals who do the work of spreading the control. Whereas, RNAi controls will require applying the control agent to areas where the target organism occurs similarly to how chemical controls are currently used. RNAi control strategy gives managers better control over where and how it is applied, whereas gene drives will spread wherever genetically modified individuals and their offspring move to. This could result in accidental escape of a gene drive from a target population to another population of the same organism where local managers may not wish for the same control to be applied (i.e. returning to the native range).
Methods for deliberate RNA-induced gene silencing were developed in the early 1990’s, and this molecular tool has since been widely used to discover complex gene functions that could not be studied before. Thousands of peer-reviewed journal articles have been published using RNAi since that discovery, and the researchers responsible for it were awarded the Nobel Prize in 2006. There are several trans-genic crops and sprays in development and being tested that use RNAi to confer resistance to agricultural pests such as corn root worm and striped flea beetle. Only recently, has its potential for controlling harmful pests and invasive species begun to be explored. This project could lead to the first application of this technology by natural resource managers to extirpate an invasive species in an area.
Study Objectives:
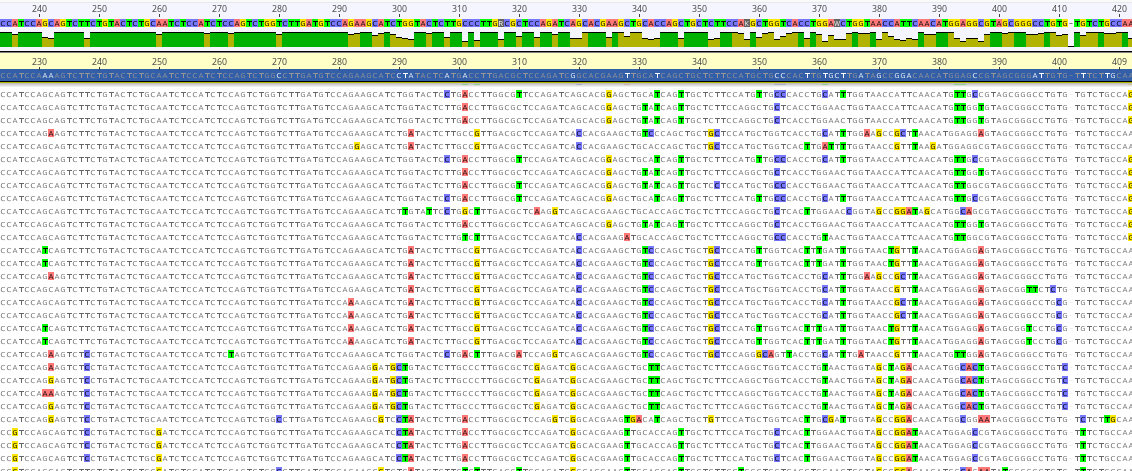
Objective 1: Obtain required genetic sequence information. To design siRNAs, we need to know the genetic sequence for genes that we can target. Therefore, we sequenced the RNA of all genes for the four invasive carp species.

Objective 2: Select target genes, design siRNAs, and develop molecular assays. We chose highly expressed genes with critical cell functions to target and designed candidate siRNAs targeting them for RNA interference. We also developed qPCR assays so that we can measure the effect of the RNA silencing.
Objective 3: Empirically test siRNA efficacy and specificity in vitro, using a cell line. We grow fish gill cells from the species we are interested in on plastic plates so that we can test the designed siRNAs for effectiveness and specificity in knocking down the target gene in only the target species.

Additional Links:
Genetic control of Grass Carp through RNA interface | U.S. Geological Survey
silver carp – Hypophthalmichthys molitrix
grass carp – Ctenopharyngodon idella
black carp – Mylopharyngodon piceus
Toward invasive mussel genetic biocontrol: Approaches, challenges, and perspectives Toward invasive mussel genetic biocontrol: Approaches, challenges, and perspectives
Assessing the suitability of YY males and ZZ females as an invasive species population control method across life histories Assessing the suitability of YY males and ZZ females as an invasive species population control method across life histories
We aim to develop control tools for eliminating invasive carps (silver carp – Hypophthalmichthys molitrix, bighead carp – H. nobilis, grass carp – Ctenopharyngodon idella, and black carp – Mylopharyngodon piceus) that use natural gene regulation mechanisms (RNA-induced gene silencing; RNA interference; RNAi) to be species-specific without non-target effects. Invasive carps cause substantial economic and ecological damage, harming valuable native fisheries while having negligible value themselves.

There are a few options available for controlling invasive carps, but their usage can vary in effectiveness or cause their own ecological damage. With chemical piscicides, there is a delicate balancing act to apply a dose that is lethal for the target organism but tolerable for others. Genetic control tools are another option that has the potential to have greater species-specificity meaning they are harmless to anything else cohabitating with the target species.
There are a couple of genetic control tools managers can use to prevent the spread of invasive carp. One strategy is to use a gene drive to spread a negative trait through a population, such as female sterility. Using one of a few molecular biology techniques (e.g. CRISPR), we can guarantee that defective alleles (version of a gene that does not work properly) will be transferred to all offspring. This means a few genetically modified individuals could be released to mate with wild-type animals (e.g. invasive carp) and change the genetic makeup of a population so that it is no longer viable resulting in population crash. Another strategy is to use RNA interference (RNAi) to selectively turn off critical genes of the target organism. This strategy takes advantage of the natural gene regulation mechanism called RNA-induced gene silencing in which small interfering RNAs (siRNAs), short pieces of RNA, guide destruction of other mRNA molecules. Targeting unique sequences that do not occur in other species allows us to turn off critical genes causing mortality while being harmless to any other species that live in the same water.
Both above strategies have advantages and disadvantages. Gene drives will require less application effort because it involves releasing a few animals who do the work of spreading the control. Whereas, RNAi controls will require applying the control agent to areas where the target organism occurs similarly to how chemical controls are currently used. RNAi control strategy gives managers better control over where and how it is applied, whereas gene drives will spread wherever genetically modified individuals and their offspring move to. This could result in accidental escape of a gene drive from a target population to another population of the same organism where local managers may not wish for the same control to be applied (i.e. returning to the native range).
Methods for deliberate RNA-induced gene silencing were developed in the early 1990’s, and this molecular tool has since been widely used to discover complex gene functions that could not be studied before. Thousands of peer-reviewed journal articles have been published using RNAi since that discovery, and the researchers responsible for it were awarded the Nobel Prize in 2006. There are several trans-genic crops and sprays in development and being tested that use RNAi to confer resistance to agricultural pests such as corn root worm and striped flea beetle. Only recently, has its potential for controlling harmful pests and invasive species begun to be explored. This project could lead to the first application of this technology by natural resource managers to extirpate an invasive species in an area.
Study Objectives:
Objective 1: Obtain required genetic sequence information. To design siRNAs, we need to know the genetic sequence for genes that we can target. Therefore, we sequenced the RNA of all genes for the four invasive carp species.

Objective 2: Select target genes, design siRNAs, and develop molecular assays. We chose highly expressed genes with critical cell functions to target and designed candidate siRNAs targeting them for RNA interference. We also developed qPCR assays so that we can measure the effect of the RNA silencing.

Objective 3: Empirically test siRNA efficacy and specificity in vitro, using a cell line. We grow fish gill cells from the species we are interested in on plastic plates so that we can test the designed siRNAs for effectiveness and specificity in knocking down the target gene in only the target species.

Additional Links:
Genetic control of Grass Carp through RNA interface | U.S. Geological Survey
silver carp – Hypophthalmichthys molitrix
grass carp – Ctenopharyngodon idella
black carp – Mylopharyngodon piceus

