A Website Tool for Predicting Triploidy in Larval Fish Spawns
Differentiating diploids from triploids at the earliest life stage possible allows for a more efficient use of resources including production time and rearing space. Thus, a reliable flow cytometric (FCM) method has been developed to discriminate triploids from diploids at the larval stage. In order to help simplify the process of differentiating triploids from diploids, we propose a simple website tool called Ploidy Predictor (available at https://warcapps.usgs.gov/gs-eco/warc/ploidy/) to help predict the number of triploid larvae from a spawn after flow cytometric processing.
The Science Issue and Relevance: Triploidy is the condition in which three chromosome sets occur in somatic cells. Triploidization is the most practical, economical, and effective method for mass production of sterile fishes. Some examples include triploid oysters Crassostrea spp., Grass Carp Ctenopharyngodon idella, and Black Carp Mylopharyngodon piceus that are commercially cultured for consumption, weed and snail control, respectively. Additionally, triploid walleye Sander vitreus, crappie Pomoxis spp., striped bass Morone saxatilis, and salmonids are stocked for recreational fishing. Triploidization limits the potential for establishment of wild populations. However, treatments used to induce triploidy often do not achieve 100% triploids in a spawn.
Differentiating diploids from triploids at the earliest life stage possible allows for a more efficient use of resources including production time and rearing space. Thus, a reliable flow cytometric (FCM) method has been developed to discriminate triploids from diploids at the larval stage. In order to help simplify the process of differentiating triploids from diploids, we propose the Ploidy Predictor tool to help predict the number of triploid larvae from a spawn after FCM processing. This tool solves the specific quadratic equations used to predict the percentage of triploid larvae in a pool of either 20 or 50 larvae of unknown ploidy. The tool will increase precision in reading the prediction graphs thereby minimizing human error in solving equations and/or interpreting the graphic displays. This tool will not only allow for exact predictions of the percentage of triploidy in larval spawns, it will simplify the process.

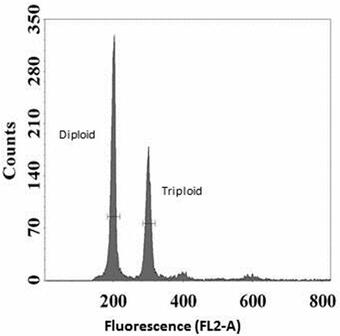
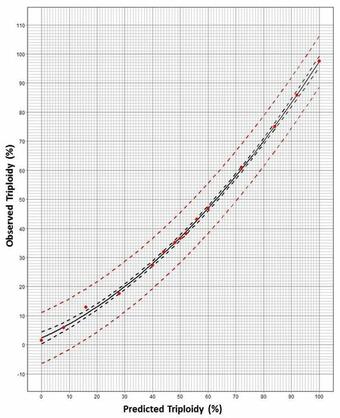
Methodology for Addressing the Issue: At 3 days post-hatch, individual Grass Carp were mechanically disassociated into single-cell suspensions. Nuclear DNA was stained with propidium iodide fluorescent dye and then analyzed by FCM to yield histograms reflecting DNA content, where triploids show nuclei with fluorescence at 1.5 times the diploid level (Fig. 1). Larvae were pooled (n = 20 or 50) per each known triploid/diploid mixture, constituting a set of 15 mixtures from 0 to 100% triploid (Fig. 1). At least 10 replications per known ploidy level were generated to determine the means and variability for the observed FCM triploidy data. Regression analyses generated the best-fitting curves, resulting in a quadratic equation specific for either the pool of 20 or 50 larvae. Thus, an accurate prediction of the proportion of triploids can be generated by following a standard larval processing and FCM analyses, coupled with using the quadratic equation (Table 1) or reading the prediction plot (Fig. 2).
Future Steps: We intend to facilitate the availability of this web-based tool (Fig. 3) to triploid producers, researchers in polyploidy, and state resource agencies. This prediction methodology can be applicable across taxa, using pools of either 20 or 50 larvae from a spawn.
Use of Prediction Plots (Fig. 2): If an observed FCM reading of the area under the triploid curve (Fig. 1) is estimated at 38% triploidy using a subsample of 50 larvae from the spawn, then the triploidy prediction from the graph is 50% in the spawn. The 95% prediction curve range is ~40 - 60%. Alternatively, quadratic equations (Table 1) can be solved for X Prediction (known) using this Y Observed reading (Fig. 3).
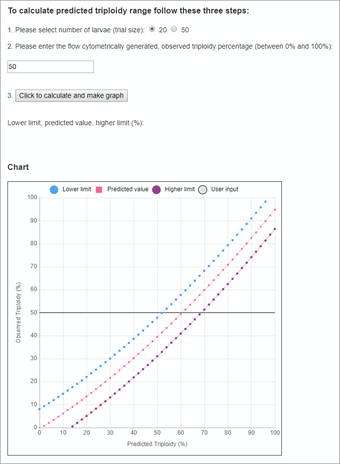
An Interactive Dialogue Box for Estimating Triploidy in a Spawn by using the online Ploidy Predictor (Fig. 3)
- Select how many fish are pooled (20 or 50) from the spawn. Enter the value in the interactive dialogue box.
- Obtain the observed triploidy value from the flow cytometrist and enter this value.
- Click the button to calculate the predicted triploidy percentage range and update the chart. The percentages for the lower calculated limit, actual predicted value, and higher calculated limit values will be displayed.
- The chart offers an alternative presentation of the numerical result. A horizontal line will highlight the observed Y value (flow cytometry data entered at step 2) across the range of predicted curve values: the 95% lower and higher prediction limits and the predicted value.

Below are other science projects associated with this project.
Determining the Ploidy and Resultant Reproductive Capability of Artificially Spawned and Wild Caught Invasive Carp
Below are publications associated with this project.
An accurate method for measuring triploidy of larval fish spawns An accurate method for measuring triploidy of larval fish spawns
Differentiating diploids from triploids at the earliest life stage possible allows for a more efficient use of resources including production time and rearing space. Thus, a reliable flow cytometric (FCM) method has been developed to discriminate triploids from diploids at the larval stage. In order to help simplify the process of differentiating triploids from diploids, we propose a simple website tool called Ploidy Predictor (available at https://warcapps.usgs.gov/gs-eco/warc/ploidy/) to help predict the number of triploid larvae from a spawn after flow cytometric processing.

The Science Issue and Relevance: Triploidy is the condition in which three chromosome sets occur in somatic cells. Triploidization is the most practical, economical, and effective method for mass production of sterile fishes. Some examples include triploid oysters Crassostrea spp., Grass Carp Ctenopharyngodon idella, and Black Carp Mylopharyngodon piceus that are commercially cultured for consumption, weed and snail control, respectively. Additionally, triploid walleye Sander vitreus, crappie Pomoxis spp., striped bass Morone saxatilis, and salmonids are stocked for recreational fishing. Triploidization limits the potential for establishment of wild populations. However, treatments used to induce triploidy often do not achieve 100% triploids in a spawn.
Differentiating diploids from triploids at the earliest life stage possible allows for a more efficient use of resources including production time and rearing space. Thus, a reliable flow cytometric (FCM) method has been developed to discriminate triploids from diploids at the larval stage. In order to help simplify the process of differentiating triploids from diploids, we propose the Ploidy Predictor tool to help predict the number of triploid larvae from a spawn after FCM processing. This tool solves the specific quadratic equations used to predict the percentage of triploid larvae in a pool of either 20 or 50 larvae of unknown ploidy. The tool will increase precision in reading the prediction graphs thereby minimizing human error in solving equations and/or interpreting the graphic displays. This tool will not only allow for exact predictions of the percentage of triploidy in larval spawns, it will simplify the process.

Methodology for Addressing the Issue: At 3 days post-hatch, individual Grass Carp were mechanically disassociated into single-cell suspensions. Nuclear DNA was stained with propidium iodide fluorescent dye and then analyzed by FCM to yield histograms reflecting DNA content, where triploids show nuclei with fluorescence at 1.5 times the diploid level (Fig. 1). Larvae were pooled (n = 20 or 50) per each known triploid/diploid mixture, constituting a set of 15 mixtures from 0 to 100% triploid (Fig. 1). At least 10 replications per known ploidy level were generated to determine the means and variability for the observed FCM triploidy data. Regression analyses generated the best-fitting curves, resulting in a quadratic equation specific for either the pool of 20 or 50 larvae. Thus, an accurate prediction of the proportion of triploids can be generated by following a standard larval processing and FCM analyses, coupled with using the quadratic equation (Table 1) or reading the prediction plot (Fig. 2).

Future Steps: We intend to facilitate the availability of this web-based tool (Fig. 3) to triploid producers, researchers in polyploidy, and state resource agencies. This prediction methodology can be applicable across taxa, using pools of either 20 or 50 larvae from a spawn.
Use of Prediction Plots (Fig. 2): If an observed FCM reading of the area under the triploid curve (Fig. 1) is estimated at 38% triploidy using a subsample of 50 larvae from the spawn, then the triploidy prediction from the graph is 50% in the spawn. The 95% prediction curve range is ~40 - 60%. Alternatively, quadratic equations (Table 1) can be solved for X Prediction (known) using this Y Observed reading (Fig. 3).

An Interactive Dialogue Box for Estimating Triploidy in a Spawn by using the online Ploidy Predictor (Fig. 3)
- Select how many fish are pooled (20 or 50) from the spawn. Enter the value in the interactive dialogue box.
- Obtain the observed triploidy value from the flow cytometrist and enter this value.
- Click the button to calculate the predicted triploidy percentage range and update the chart. The percentages for the lower calculated limit, actual predicted value, and higher calculated limit values will be displayed.
- The chart offers an alternative presentation of the numerical result. A horizontal line will highlight the observed Y value (flow cytometry data entered at step 2) across the range of predicted curve values: the 95% lower and higher prediction limits and the predicted value.

Below are other science projects associated with this project.
Determining the Ploidy and Resultant Reproductive Capability of Artificially Spawned and Wild Caught Invasive Carp
Below are publications associated with this project.


